PH Values Presentation
| Introduction to pH Values | ||
|---|---|---|
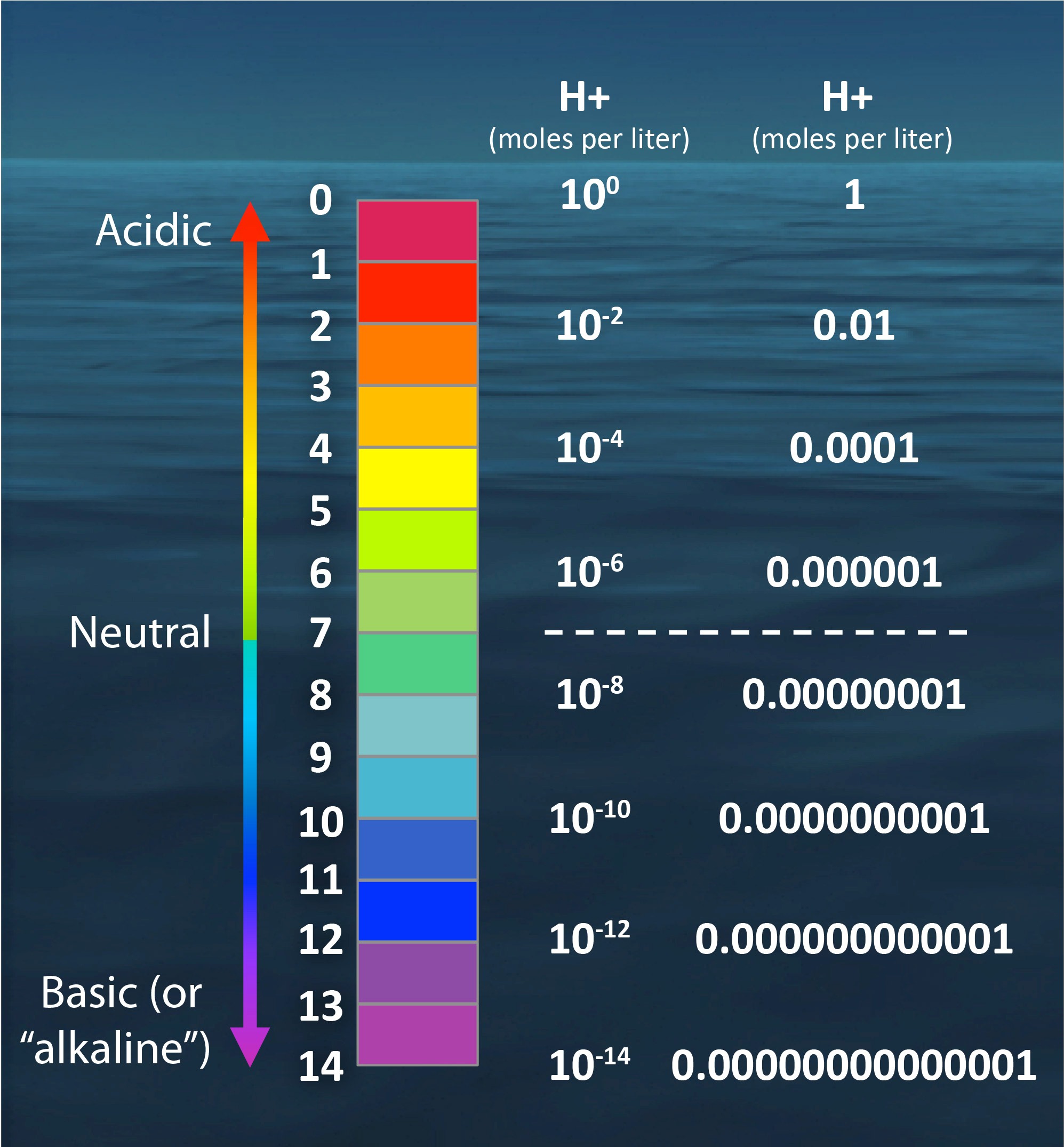
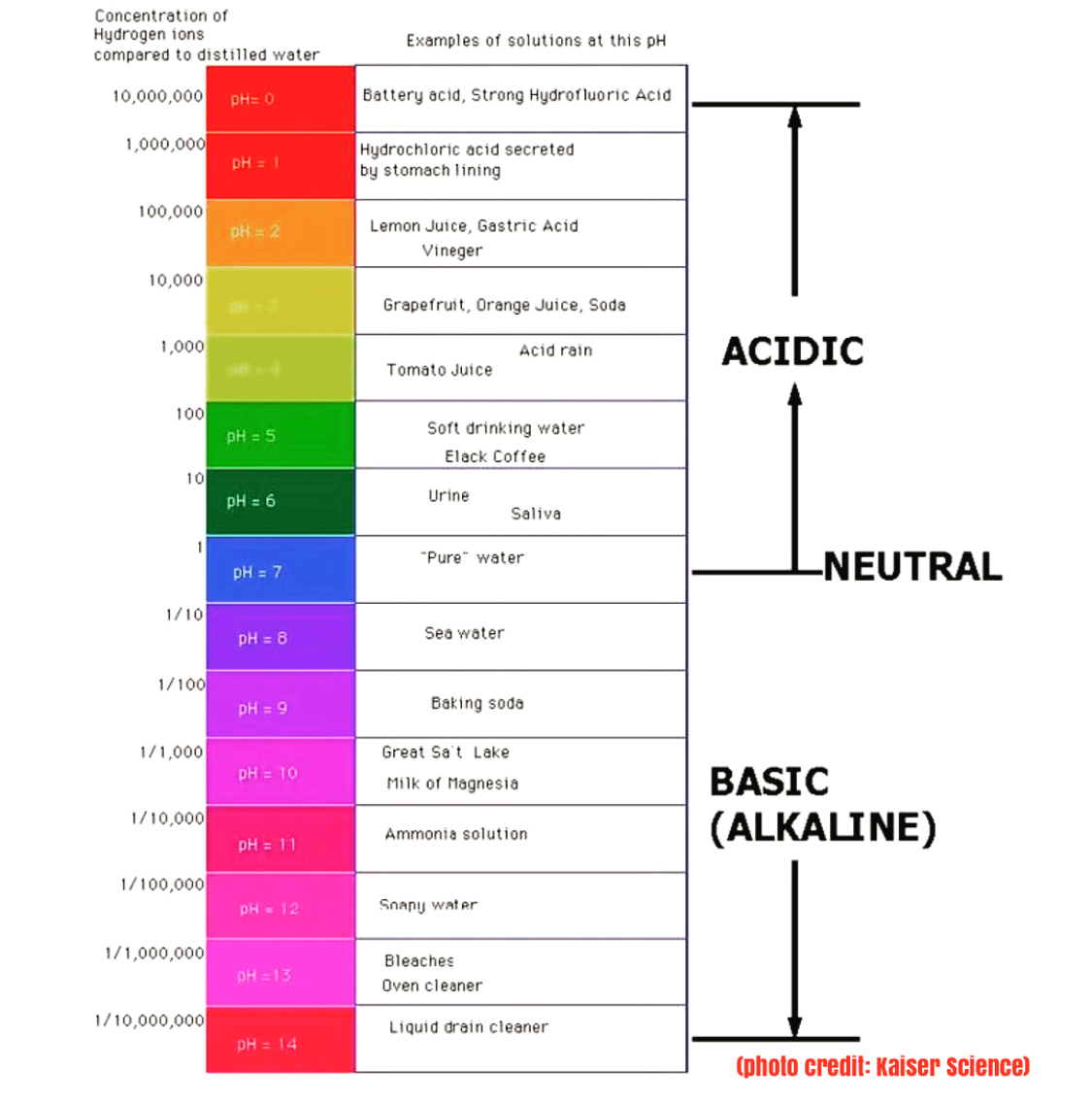
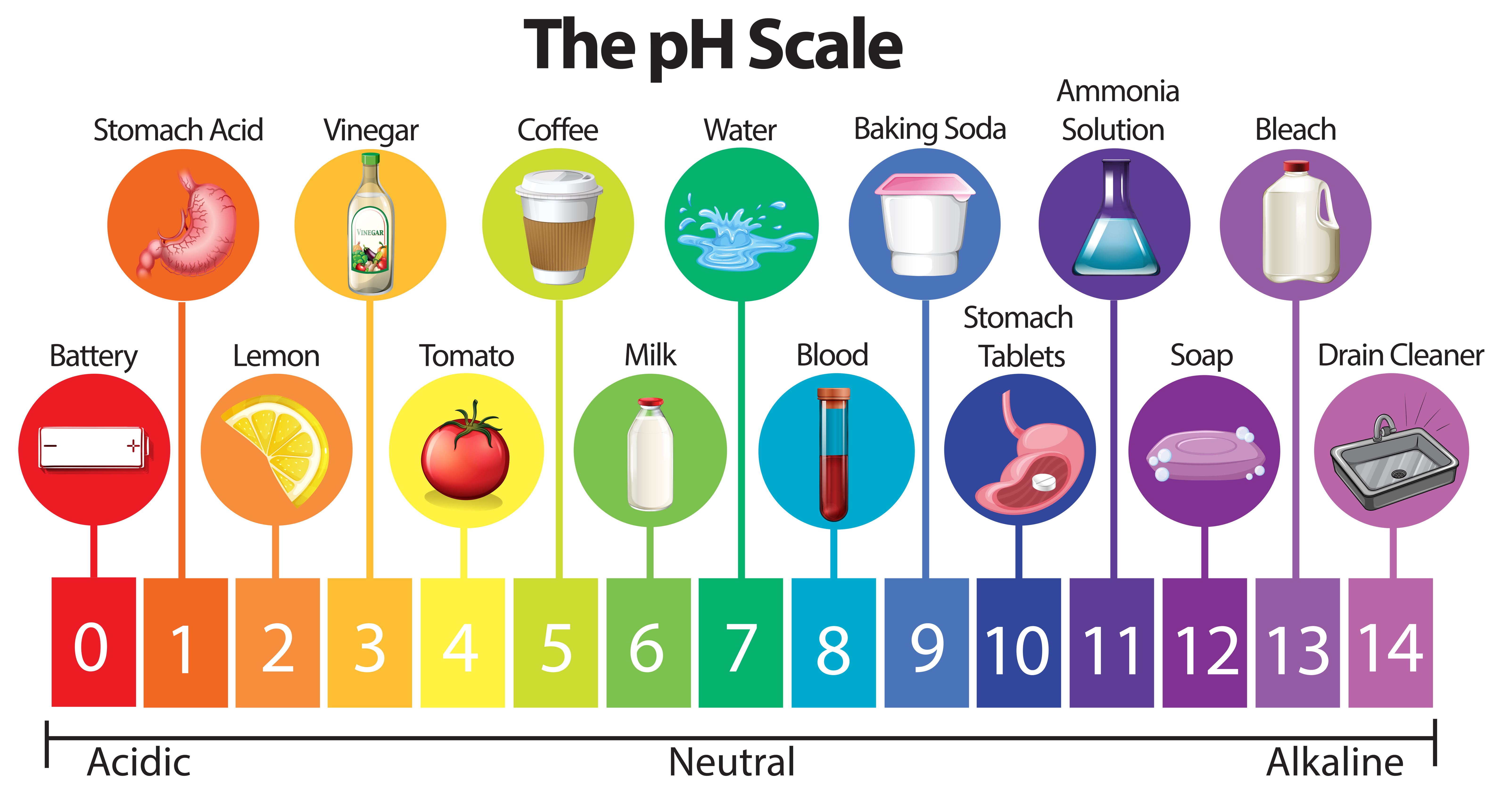
| pH values are a measure of acidity or alkalinity in a solution. The pH scale ranges from 0 to 14. A pH value of 7 is considered neutral. | ||
| 1 | ||
| Understanding the pH Scale | ||
|---|---|---|
| pH values below 7 indicate acidity, with lower numbers representing stronger acidity. pH values above 7 indicate alkalinity, with higher numbers representing stronger alkalinity. Each unit on the pH scale represents a tenfold difference in acidity or alkalinity. | ||
| 2 | ||
| Importance of pH in Everyday Life | ||
|---|---|---|
| pH plays a crucial role in the environment, affecting the health of ecosystems and aquatic life. In agriculture, pH values determine soil fertility and the availability of nutrients to plants. pH levels also impact our health, influencing the effectiveness of medications and the functioning of our digestive system. | ||
| 3 | ||
| Measuring pH | ||
|---|---|---|
| pH can be measured using pH paper strips or electronic pH meters. pH paper strips change color according to the acidity or alkalinity of a solution. Electronic pH meters provide more accurate readings and are commonly used in laboratories and industrial settings. | ||
| 4 | ||
| Acidic Solutions | ||
|---|---|---|
| Acidic solutions have pH values below 7. Examples of acidic substances include lemon juice, vinegar, and battery acid. Acidic solutions can cause corrosion and irritation. | ||
| 5 | ||
| Neutral Solutions | ||
|---|---|---|
| Neutral solutions have a pH value of 7. Pure water is considered neutral. Neutral solutions are neither acidic nor alkaline. | ||
| 6 | ||
| Alkaline Solutions | ||
|---|---|---|
| Alkaline solutions have pH values above 7. Examples of alkaline substances include baking soda, ammonia, and bleach. Alkaline solutions can be corrosive and harmful if handled improperly. | ||
| 7 | ||
| pH in Household Products | ||
|---|---|---|
| Many household products, such as detergents and cleaning agents, have specific pH levels. pH values in these products are designed to optimize their effectiveness. Understanding pH can help us choose the right products for various cleaning tasks. | ||
| 8 | ||
| pH Regulation in the Body | ||
|---|---|---|
| Our bodies maintain a specific pH balance to ensure proper functioning. Blood pH, for example, is tightly regulated around 7.4. Imbalances in pH can lead to health issues and even be life-threatening. | ||
| 9 | ||
| Conclusion | ||
|---|---|---|
| pH values are a fundamental concept in chemistry and have practical applications in various aspects of our lives. Understanding pH allows us to make informed decisions about cleaning products, agricultural practices, and even our own health. By appreciating the importance of pH, we can better appreciate the delicate balance of our surroundings. | ||
| 10 | ||