Toxicology Studies Presentation
| Introduction to Toxicology Studies | ||
|---|---|---|
| Toxicology studies evaluate the effects of toxic substances on living organisms. These studies help identify potential hazards and assess the risks associated with exposure to these substances. Toxicology studies are vital for determining safe levels of exposure and developing appropriate safety guidelines. | ||
| 1 | ||
| Types of Toxicology Studies | ||
|---|---|---|
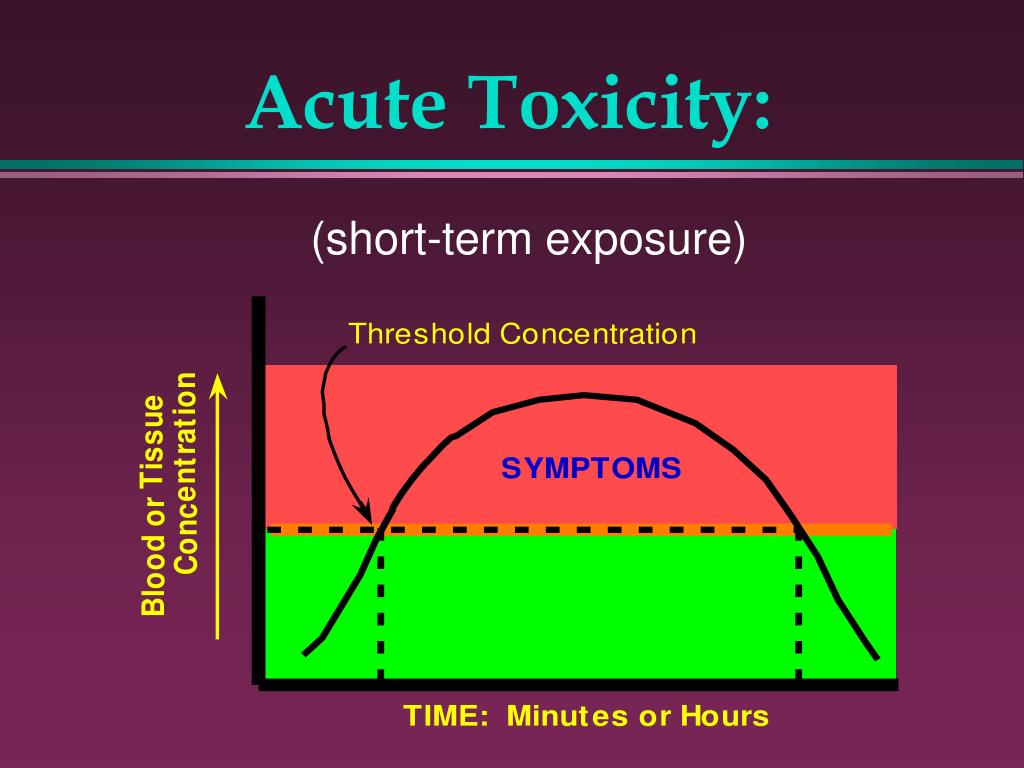
| Acute Toxicity Studies: Determine the effects of a single exposure to a toxic substance over a short period. Subchronic Toxicity Studies: Assess the effects of repeated exposure to a toxic substance for a few weeks or months. Chronic Toxicity Studies: Evaluate the effects of long-term exposure to a toxic substance. | ||
| 2 | ||
| Study Design and Methodology | ||
|---|---|---|
| In vitro Studies: Conducted in a controlled laboratory setting using isolated cells or tissues. In vivo Studies: Conducted on live animals to evaluate the systemic effects of a toxic substance. Epidemiological Studies: Analyze the effects of toxic substances on human populations through observation and data analysis. | ||
| 3 | ||
| Measurement and Analysis | ||
|---|---|---|
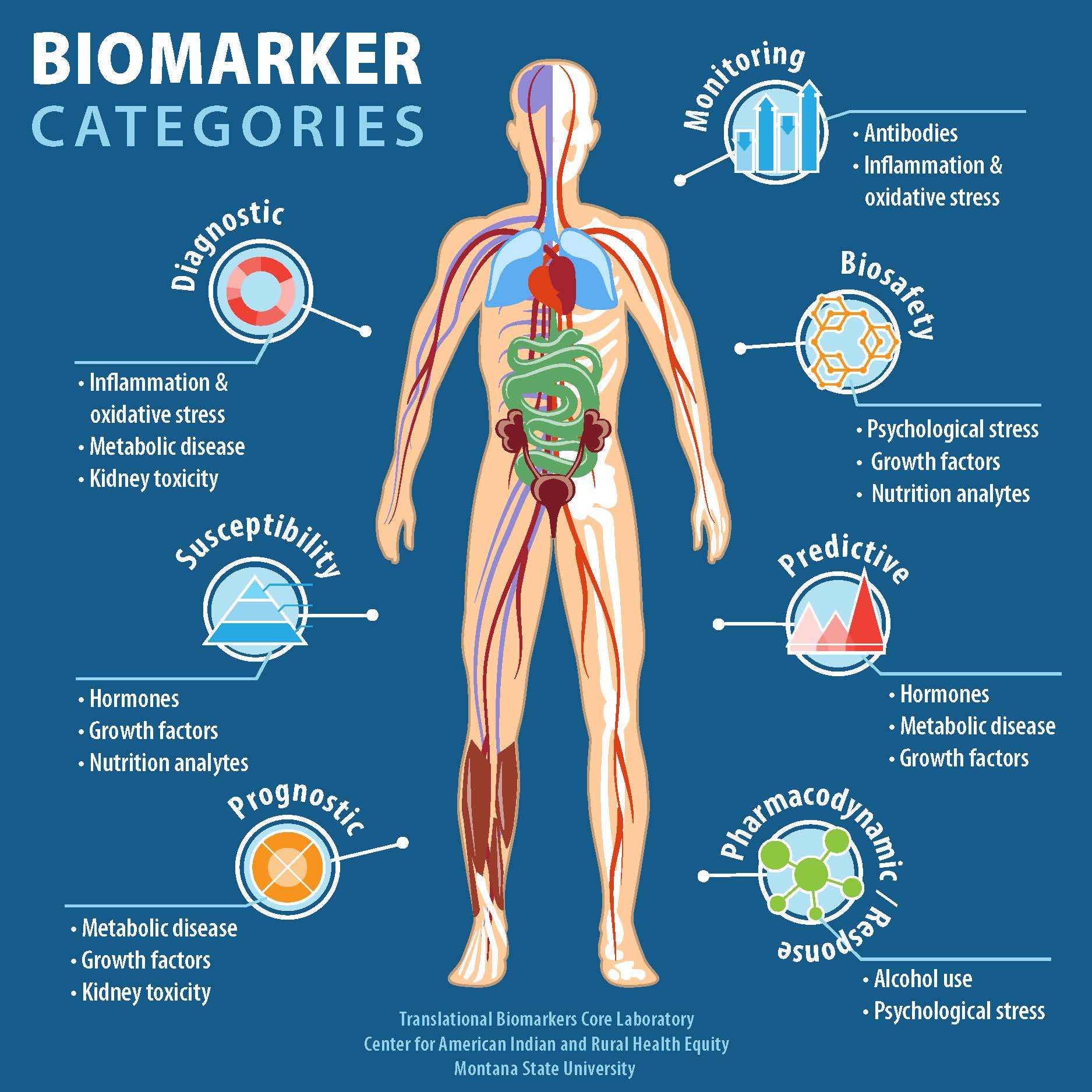
| Biomarkers: Used to measure the presence or levels of toxic substances or their metabolites in biological samples. Dose-Response Assessment: Determines the relationship between the dose of a toxic substance and the response or adverse effect. Statistical Analysis: Used to interpret study results and determine significance and confidence levels. | ||
| 4 | ||
| Study Endpoints and Parameters | ||
|---|---|---|
| Acute Toxicity: Assessing mortality, clinical signs, and organ damage following a single exposure. Subchronic and Chronic Toxicity: Monitoring changes in body weight, organ function, hematological and biochemical parameters, and histopathological examination. Reproductive and Developmental Toxicity: Evaluating effects on fertility, pregnancy, and the development of offspring. | ||
| 5 | ||
| Regulatory Considerations | ||
|---|---|---|
| Toxicology studies are essential for regulatory agencies to establish safety standards for various industries. Studies support the approval and registration of pharmaceuticals, pesticides, chemicals, and consumer products. Toxicology data is crucial in risk assessment and decision-making processes. | ||
| 6 | ||
| Importance of Toxicology Studies | ||
|---|---|---|
| Protecting human health and safety by identifying potential toxic hazards. Supporting the development of safe and effective therapies and treatments. Informing regulatory decisions and setting exposure limits to mitigate risks. | ||
| 7 | ||
| Limitations and Challenges | ||
|---|---|---|
| Ethical considerations concerning the use of animals in toxicology studies. Variability in the response to toxic substances among different species. Difficulty in accurately predicting human response based solely on animal studies. | ||
| 8 | ||
| Future Directions | ||
|---|---|---|
| Increased emphasis on non-animal testing methods, such as in vitro models and computer simulations. Advancements in biomarker identification and analysis techniques. Integration of omics technologies (genomics, proteomics, metabolomics) for a comprehensive understanding of toxicological mechanisms. | ||
| 9 | ||
| Summary | ||
|---|---|---|
| Toxicology studies are crucial for assessing the safety and risks associated with exposure to toxic substances. Various study designs, measurement techniques, and analysis methods are employed to evaluate toxic effects. These studies play a pivotal role in protecting human health, supporting regulatory decisions, and advancing scientific knowledge. | ||
| 10 | ||
| References (download PPTX file for details) | ||
|---|---|---|
| Casarett & Doull's Toxicology: The Basic Scie... Eighth Edition... Hayes' Principles and Methods of Toxicology... |  | |
| 11 | ||