Structure Of Atom Presentation
| Introduction to the Structure of the Atom | ||
|---|---|---|
| The atom is the basic building block of matter. Atoms are made up of three subatomic particles: protons, neutrons, and electrons. Understanding the structure of the atom is crucial to understanding the properties and behavior of matter. | ||
| 1 | ||
| Subatomic Particles | ||
|---|---|---|
| Protons are positively charged particles found in the nucleus of an atom. Neutrons are neutral particles found in the nucleus of an atom. Electrons are negatively charged particles that orbit the nucleus in energy levels. | ||
| 2 | ||
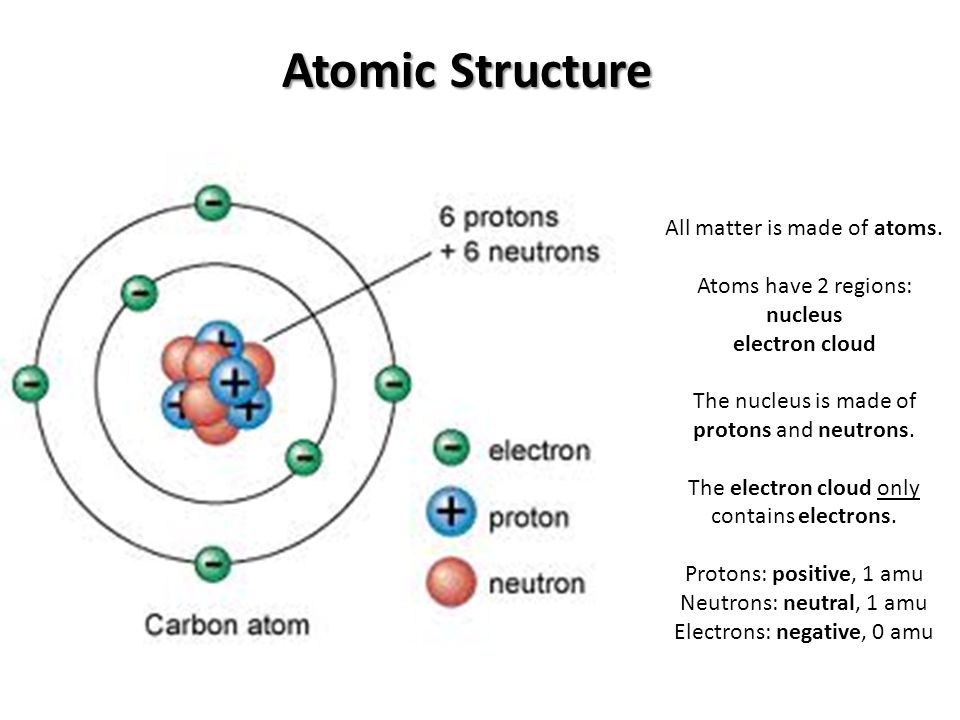
| Nucleus | ||
|---|---|---|
| The nucleus is the central part of an atom, containing protons and neutrons. Protons have a positive charge and provide the atomic number of an element. Neutrons have no charge and contribute to the stability of the nucleus. | ||
| 3 | ||
| Electron Cloud | ||
|---|---|---|
| Electrons are located outside the nucleus in electron clouds or energy levels. Electrons occupy specific shells or orbitals depending on their energy level. The further an electron is from the nucleus, the higher its energy level. | ||
| 4 | ||
| Energy Levels | ||
|---|---|---|
| Energy levels are represented by the principle quantum number (n). The first energy level (n=1) is closest to the nucleus and can hold up to 2 electrons. Each subsequent energy level can hold more electrons. | ||
| 5 | ||
| Orbitals and Subshells | ||
|---|---|---|
| Orbitals are regions within an energy level where electrons are most likely to be found. Orbitals are further divided into subshells, represented by the azimuthal quantum number (l). Subshells have different shapes and can hold different numbers of electrons. | ||
| 6 | ||
| Electron Configuration | ||
|---|---|---|
| Electron configuration describes the arrangement of electrons in an atom. Aufbau principle states that electrons fill the lowest energy level first before moving to higher energy levels. The Pauli exclusion principle states that each orbital can hold a maximum of 2 electrons with opposite spins. | ||
| 7 | ||
| Valence Electrons | ||
|---|---|---|
| Valence electrons are the electrons in the outermost energy level of an atom. Valence electrons determine the chemical properties and reactivity of an element. Elements in the same group of the periodic table have the same number of valence electrons. | ||
| 8 | ||
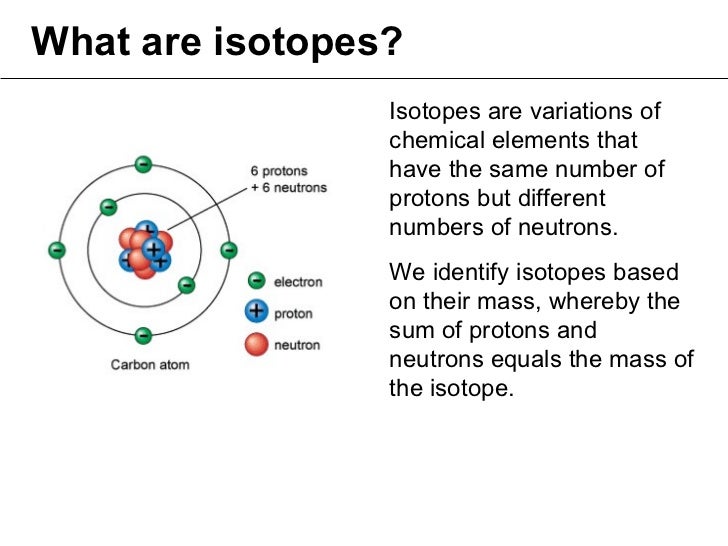
| Isotopes | ||
|---|---|---|
| Isotopes are atoms of the same element with different numbers of neutrons. Isotopes have the same number of protons and electrons but different mass numbers. Isotopes can have different physical and chemical properties. | ||
| 9 | ||
| Conclusion | ||
|---|---|---|
| The structure of the atom is a fundamental concept in chemistry and physics. Understanding the structure of the atom helps explain the behavior of matter and the properties of elements. Advances in atomic structure have led to significant scientific discoveries and technological advancements. | ||
| 10 | ||
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)