Potentiometric -position Presentation
| Introduction to Potentiometric -position | ||
|---|---|---|
| Potentiometric -position is a technique used to measure the -position of a solution or substance. It is based on the principle of measuring the potential difference between two electrodes immersed in the solution. This technique is widely used in various fields such as environmental monitoring, pharmaceutical analysis, and chemical research. | ||
| 1 | ||
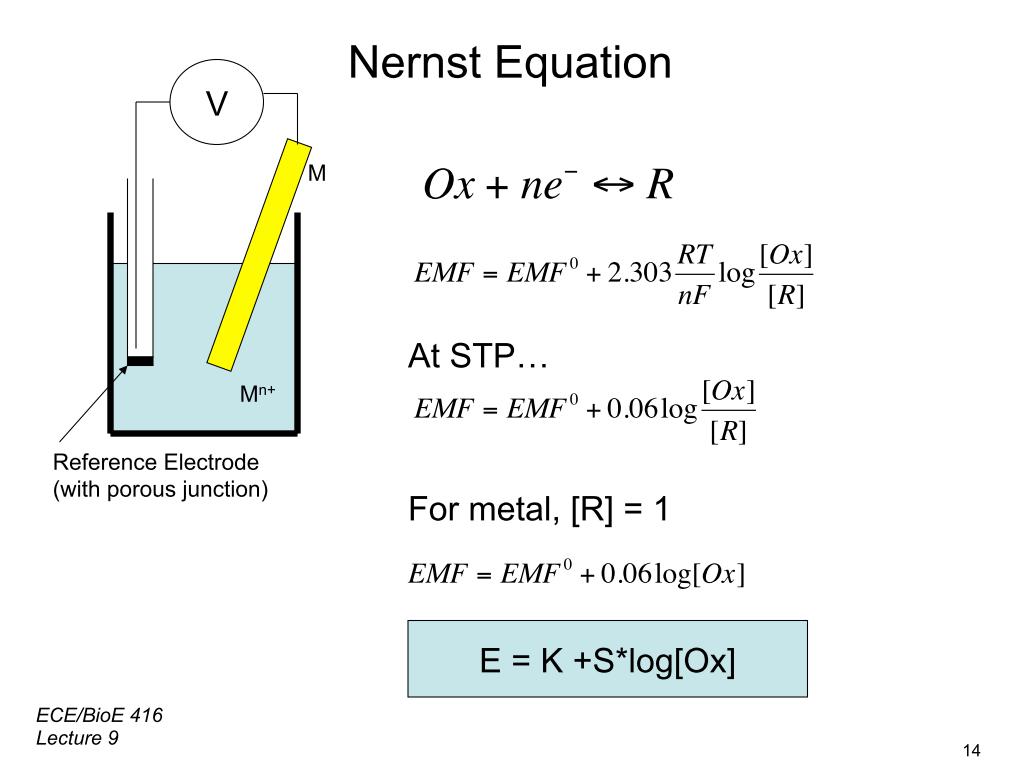
| Principle of Potentiometric -position | ||
|---|---|---|
| Potentiometric -position is based on the Nernst equation, which relates the potential difference to the concentration of -ions. The potential difference is measured using a high impedance voltmeter connected to the electrodes. The potential difference is directly proportional to the logarithm of the -ion concentration according to the Nernst equation. | ||
| 2 | ||
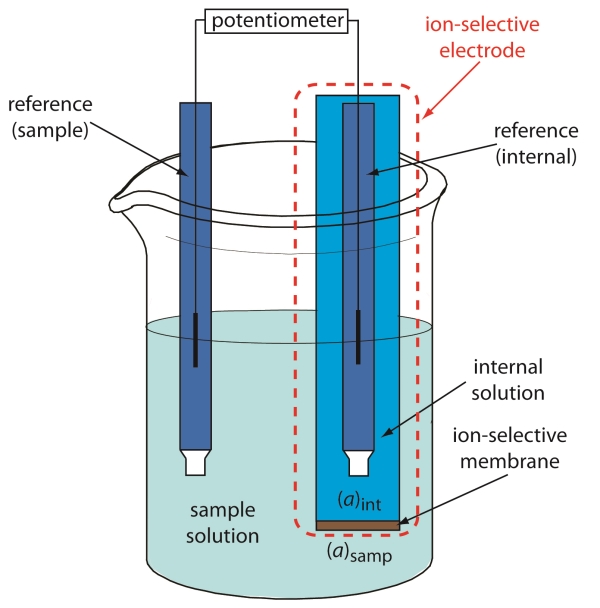
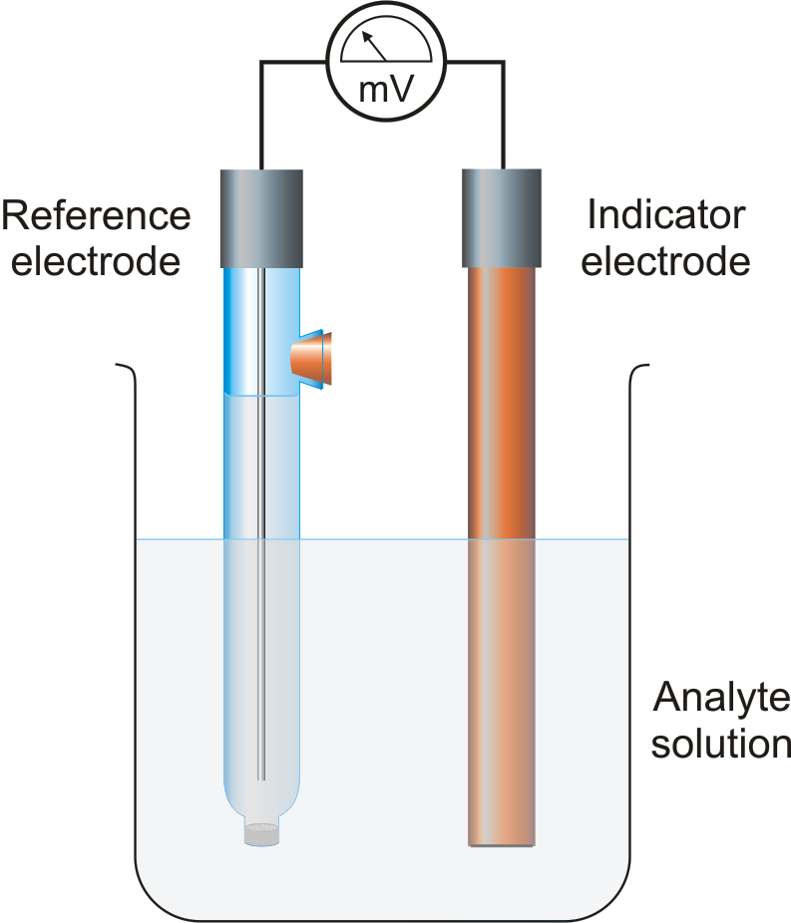
| Electrodes Used in Potentiometric -position | ||
|---|---|---|
| The two main types of electrodes used in potentiometric -position are the reference electrode and the indicator electrode. The reference electrode provides a stable and known potential against which the potential of the indicator electrode is measured. The indicator electrode is selective to the -ion of interest and its potential varies with the concentration of -ions in the solution. | ||
| 3 | ||
| Types of Potentiometric -position | ||
|---|---|---|
| Direct potentiometry measures the potential difference between a reference electrode and an indicator electrode directly in the sample solution. Ion-selective electrodes (ISEs) are commonly used in direct potentiometry for specific -ions such as pH, fluoride, chloride, etc. Indirect potentiometry involves the use of a titration setup where the potential difference is measured during a titration process. | ||
| 4 | ||
| Applications of Potentiometric -position | ||
|---|---|---|
| Potentiometric -position is extensively used in clinical laboratories for the measurement of various ions in body fluids such as blood, urine, and saliva. It is used in environmental monitoring to measure the -ion concentration in water bodies to assess water quality. In the pharmaceutical industry, potentiometric -position is used for drug analysis, dosage form development, and quality control. | ||
| 5 | ||
| Advantages of Potentiometric -position | ||
|---|---|---|
| Potentiometric -position offers high accuracy and precision in -ion concentration measurements. It is a non-destructive technique, allowing repeated measurements on the same sample. It has a wide dynamic range, making it suitable for measuring both low and high -ion concentrations. | ||
| 6 | ||
| Limitations of Potentiometric -position | ||
|---|---|---|
| Potentiometric -position requires careful calibration and maintenance of electrodes to ensure accurate results. Interference from other ions or substances in the sample can affect the accuracy of measurements. It is a relatively slow technique compared to other -position methods. | ||
| 7 | ||
| Factors Affecting Potentiometric -position | ||
|---|---|---|
| Temperature can affect the potential difference and should be controlled and accounted for in measurements. The presence of complexing agents, surfactants, or other interfering substances can affect the selectivity and response of the indicator electrode. Electrode drift can occur over time, requiring periodic calibration and electrode replacement. | ||
| 8 | ||
| Examples of Potentiometric -position Setups | ||
|---|---|---|
| A pH meter with a pH electrode is a common potentiometric -position setup used for measuring the pH of a solution. A fluoride ion-selective electrode connected to a voltmeter can be used to measure the fluoride concentration in water. A potentiometric titration setup with a reference electrode, indicator electrode, and a burette is used for acid-base titrations. | ||
| 9 | ||
| Summary of Potentiometric -position | ||
|---|---|---|
| Potentiometric -position is a powerful technique for measuring -ion concentrations in various applications. It is based on the Nernst equation and utilizes electrodes to measure the potential difference. This technique offers high accuracy, non-destructive measurements, and a wide dynamic range. | ||
| 10 | ||
| References (download PPTX file for details) | ||
|---|---|---|
| Smith, J. (2018). Principles and Applications... Johnson, A. B. (2019). Advanced Techniques in... Garcia, D., & Martinez, C. (2020). Potentiome... |  | |
| 11 | ||