Photoelectric Effect Presentation
| Introduction | ||
|---|---|---|
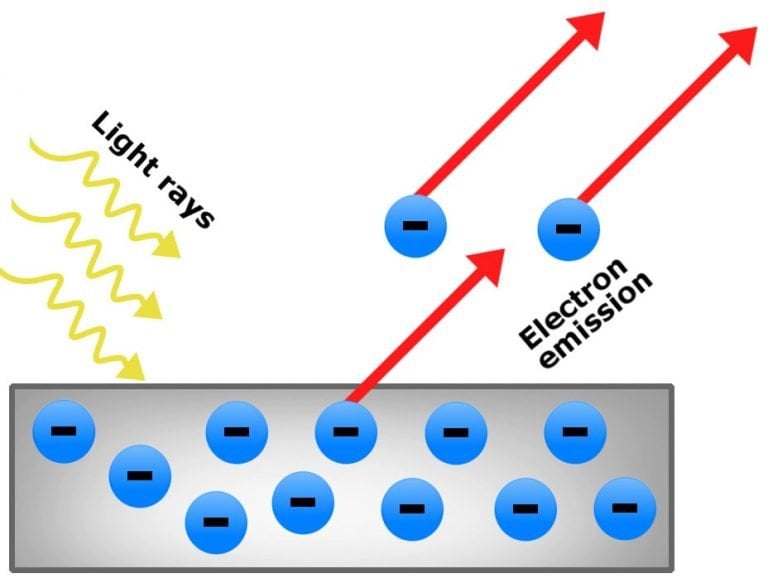
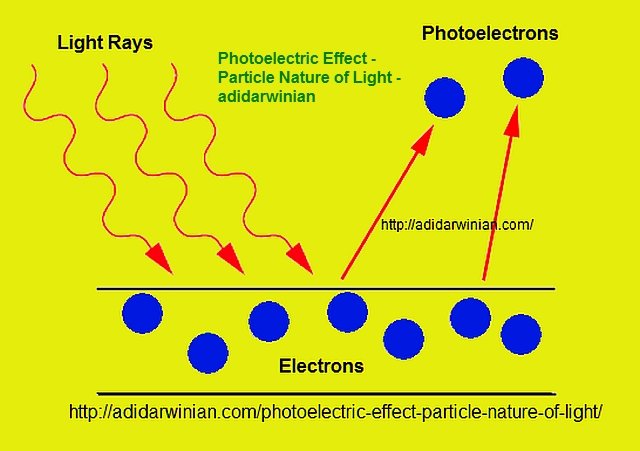
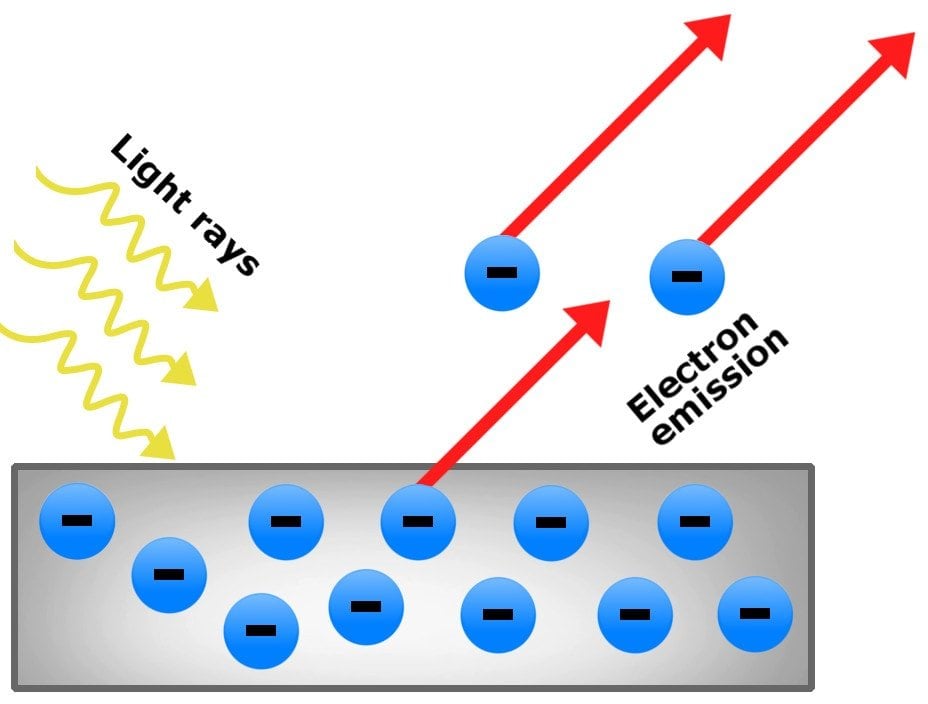
| The photoelectric effect is the phenomenon where electrons are emitted from a material when it is exposed to light. It was first discovered by Heinrich Hertz in 1887. The effect plays a crucial role in understanding the behavior of light and electrons. | ||
| 1 | ||
| Key Observations | ||
|---|---|---|
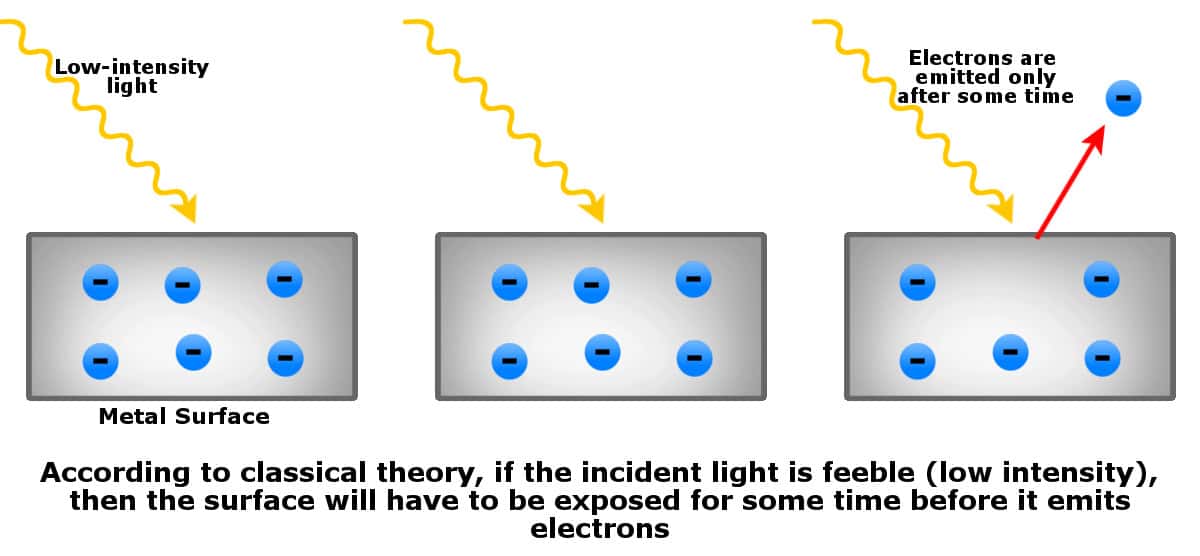
| Electrons are only emitted when the frequency of light exceeds a certain threshold value, known as the threshold frequency. The energy of the emitted electrons increases with the frequency of light. Increasing the intensity of light does not affect the kinetic energy of emitted electrons, but it increases the number of emitted electrons. | ||
| 2 | ||
| Explanation | ||
|---|---|---|
| The photoelectric effect can be explained using the particle nature of light, as proposed by Albert Einstein. Einstein introduced the concept of photons – discrete packets of energy carried by light. When a photon strikes a material, it transfers its energy to an electron, which can then overcome the binding energy and be emitted. | ||
| 3 | ||
| Quantum Nature | ||
|---|---|---|
| The photoelectric effect supports the idea that light possesses both particle and wave-like properties. The fact that electrons are emitted only when the light frequency exceeds the threshold frequency suggests a quantized energy transfer. Einstein's explanation of the photoelectric effect laid the foundation for the development of quantum mechanics. | ||
| 4 | ||
| Applications | ||
|---|---|---|
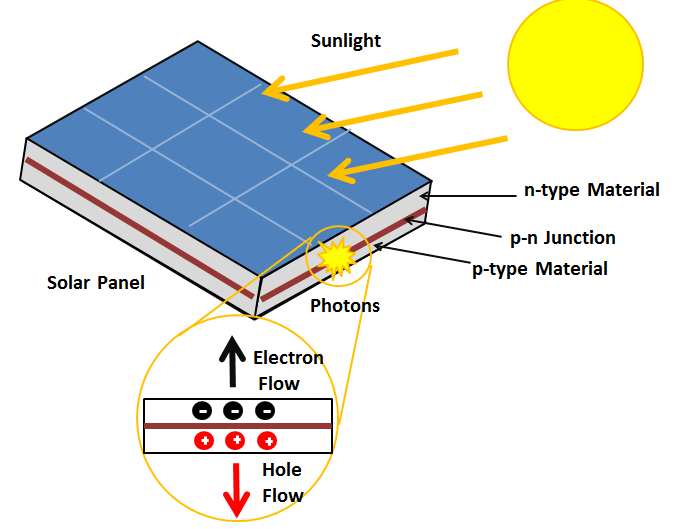
| The photoelectric effect is utilized in photovoltaic cells to convert sunlight into electric energy. It is widely used in digital cameras, image sensors, and solar panels. Photoelectric sensors are used in various industrial and scientific applications, such as motion detection and particle counting. | ||
| 5 | ||
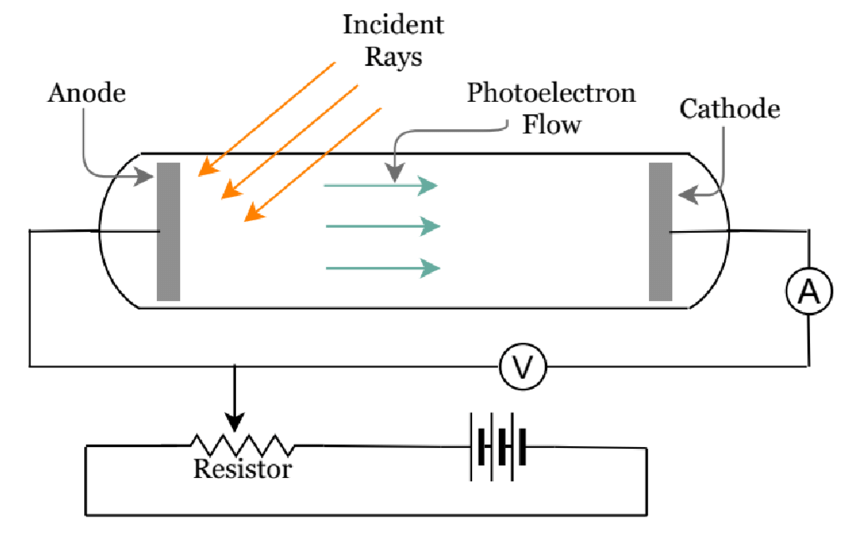
| Experimental Setup | ||
|---|---|---|
| To observe the photoelectric effect, a vacuum tube is used, containing a metal plate (cathode) and a positively charged plate (anode). When light is incident on the cathode, electrons are emitted and accelerated toward the anode. The resulting current can be measured and analyzed. | ||
| 6 | ||
| Einstein's Nobel Prize | ||
|---|---|---|
| Albert Einstein was awarded the Nobel Prize in Physics in 1921 for his explanation of the photoelectric effect. His work revolutionized the understanding of light and laid the foundation for the development of quantum mechanics. Einstein's contributions to the photoelectric effect paved the way for future advancements in physics. | ||
| 7 | ||
| Limitations | ||
|---|---|---|
| The photoelectric effect does not occur in all materials. Certain metals and semiconductors are most susceptible to it. The effect is not observed for light below a certain frequency, even if the intensity is high. The kinetic energy of emitted electrons is independent of the intensity of light, only depending on its frequency. | ||
| 8 | ||
| Significance | ||
|---|---|---|
| The photoelectric effect provided experimental evidence for the quantization of energy and the particle-wave duality of light. It played a crucial role in the development of quantum mechanics and the understanding of the behavior of electrons. The applications of the photoelectric effect in various technologies have transformed numerous industries. | ||
| 9 | ||
| Conclusion | ||
|---|---|---|
| The photoelectric effect is a fundamental phenomenon where electrons are emitted from a material when exposed to light. It supports the particle-wave duality of light and the quantization of energy. The effect has significant applications in energy conversion, technology, and scientific research. | ||
| 10 | ||
| References (download PPTX file for details) | ||
|---|---|---|
| Einstein, A. (1905). Über einen die Erzeugung... Einstein, A. (1921). The Nobel Prize in Physi... NobelPrize.org. Retrieved from https:// www.n... |  | |
| 11 | ||