Pharma Company Internship On Quality Control Department Presentation
| Introduction | ||
|---|---|---|
| Pharma Company Internship on Quality Control Department. Overview of the internship program. Importance of quality control in the pharmaceutical industry. | ||
| 1 | ||
| Internship Objectives | ||
|---|---|---|
| Gain hands-on experience in quality control processes. Understand the regulatory requirements and guidelines. Contribute to the improvement of product quality and safety. | ||
| 2 | ||
| Department Overview | ||
|---|---|---|
| Role of the quality control department in a pharma company. Responsibilities of the QC team. Collaboration with other departments like manufacturing and R&D. |  | |
| 3 | ||
| Key Activities | ||
|---|---|---|
| Perform routine testing of raw materials, intermediates, and finished products. Conduct stability studies to assess product shelf-life. Analyze samples using various techniques like HPLC, GC, and spectrophotometry. | ||
| 4 | ||
| Quality Control Techniques | ||
|---|---|---|
| Use of analytical instruments for accurate and precise measurements. Microbiological testing to ensure product sterility. Identification of impurities and contaminants in samples. | ||
| 5 | ||
| Documentation and Record-Keeping | ||
|---|---|---|
| Importance of maintaining accurate and complete records. Adherence to Good Documentation Practices (GDP). Documentation of test results, procedures, and instrument calibration. | ||
| 6 | ||
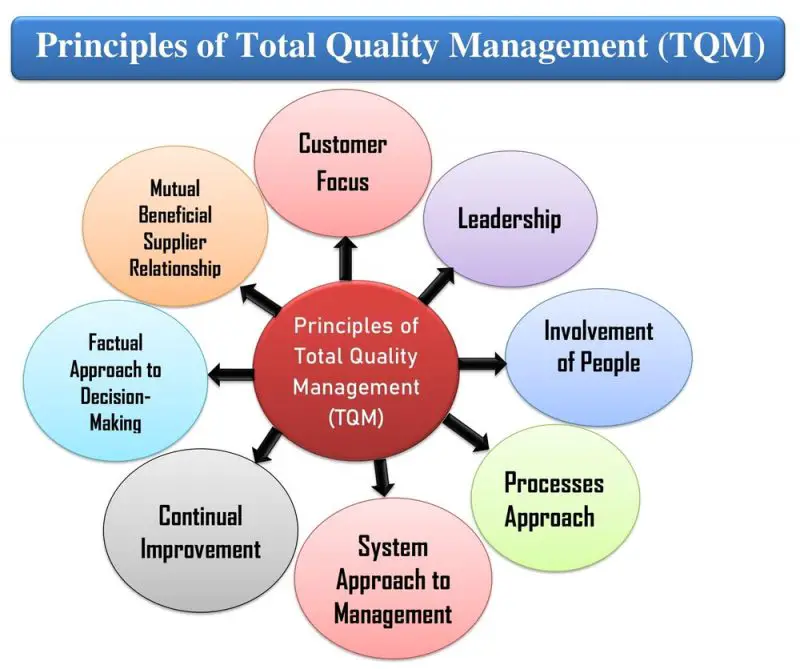
| Compliance and Regulatory Requirements | ||
|---|---|---|
| Understanding and implementation of Good Manufacturing Practices (GMP). Compliance with regulatory bodies such as FDA, EMA, and WHO. Adherence to Standard Operating Procedures (SOPs) and protocols. | ||
| 7 | ||
| Quality Control Challenges | ||
|---|---|---|
| Ensuring accuracy and reliability of test results. Handling and disposal of hazardous materials. Troubleshooting instrument malfunctions and deviations. | ||
| 8 | ||
| Learning Opportunities | ||
|---|---|---|
| Exposure to various analytical techniques and instruments. Understanding of quality control principles and methodologies. Collaboration with cross-functional teams for problem-solving. | ||
| 9 | ||
| Conclusion | ||
|---|---|---|
| Pharma company internship on quality control provides valuable industry experience. Opportunity to contribute to product quality and safety. Launchpad for future career opportunities in the pharmaceutical industry. | ||
| 10 | ||