Joule Thomson Effect Presentation
| Introduction to the Joule Thomson Effect | ||
|---|---|---|
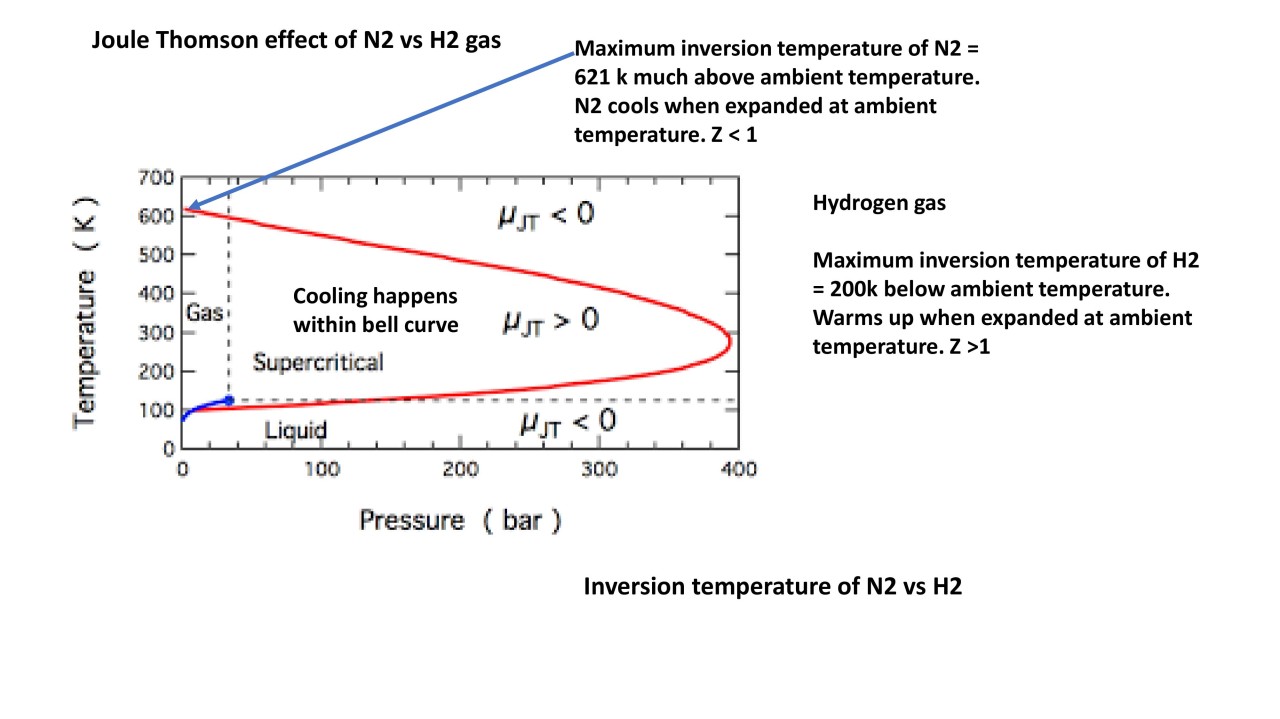
| The Joule Thomson Effect refers to the change in temperature of a gas when it is allowed to expand through a throttling valve or porous plug. Named after James Prescott Joule and William Thomson (Lord Kelvin), who first studied and described this phenomenon. The effect is commonly observed in various applications such as refrigeration, liquefied gas production, and natural gas processing. | ||
| 1 | ||
| Understanding the Joule Thomson Coefficient | ||
|---|---|---|
| The Joule Thomson Coefficient (μ) is used to quantify the magnitude of the temperature change during the expansion process. It is defined as the rate of change of temperature with respect to pressure at constant enthalpy. The coefficient can be positive, negative, or zero, depending on the nature of the gas and its initial conditions. | ||
| 2 | ||
| Factors Affecting Joule Thomson Effect | ||
|---|---|---|
| The magnitude of the temperature change depends on the pressure and temperature of the gas before expansion. Gases with a high initial pressure and low initial temperature tend to experience a significant decrease in temperature during expansion. The nature of the gas molecules, intermolecular forces, and the specific heat capacity also influence the Joule Thomson Effect. | ||
| 3 | ||
| Applications of the Joule Thomson Effect | ||
|---|---|---|
| Refrigeration: The Joule Thomson Effect is utilized in refrigeration systems to cool gases, liquids, or solids. Liquefied Gas Production: The expansion of compressed gases through a throttling valve allows for the production of liquefied gases such as liquefied petroleum gas (LPG). Natural Gas Processing: The Joule Thomson Effect is exploited to separate natural gas components based on their boiling points, enabling the extraction of valuable gases such as methane. |  | |
| 4 | ||
| Limitations and Challenges | ||
|---|---|---|
| The Joule Thomson Effect can only be observed in gases, as it requires the free movement of gas molecules. It is essential to consider safety precautions, as the temperature drop during expansion can lead to the formation of ice plugs or freezing of equipment. Accurate prediction and control of the Joule Thomson Effect require detailed thermodynamic models and experimental data, posing challenges in practical applications. Note: Each slide should be accompanied by relevant visuals, diagrams, or equations to enhance understanding and engagement. |  | |
| 5 | ||