Informed Consent Process And Procedures Presentation
| Introduction | ||
|---|---|---|
| Informed consent is a crucial part of the research and medical process. It ensures that individuals have all the necessary information to make an informed decision. Informed consent protects the rights and well-being of participants. | ||
| 1 | ||
| Definition | ||
|---|---|---|
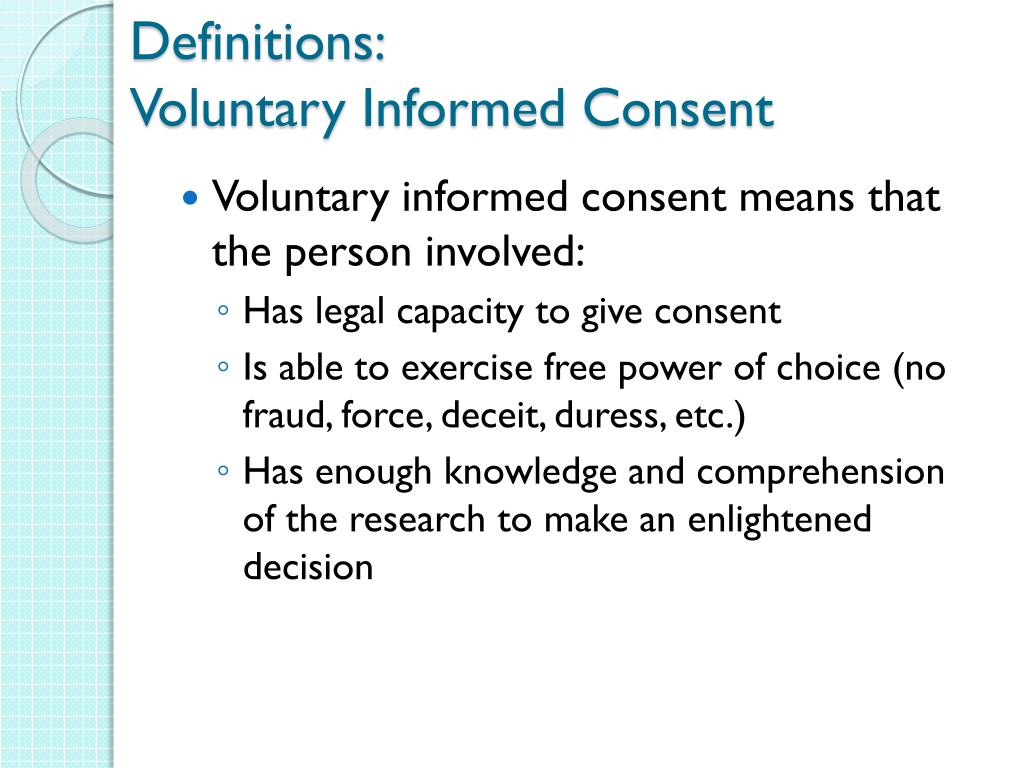
| Informed consent is the voluntary agreement of an individual to participate in research or medical procedures. It is based on the understanding and comprehension of the purpose, risks, benefits, and alternatives involved. Informed consent is an ongoing process that may be revisited at any time during the research or medical treatment. | ||
| 2 | ||
| Key Elements | ||
|---|---|---|
| Full disclosure of information: Participants must receive clear and complete information about the research or medical procedure. Capacity of understanding: Participants should possess the mental and legal capacity to comprehend and make decisions. Voluntary participation: Participants must be free from coercion, manipulation, or undue influence to give consent. | ||
| 3 | ||
| Documentation | ||
|---|---|---|
| Written consent form: A written document is used to outline the information provided and the participant's agreement. Date and signature: Participants must sign and date the form to indicate their consent. Copies for participants: Participants should receive a copy of the signed consent form for their records. | ||
| 4 | ||
| Process | ||
|---|---|---|
| Initial discussion: Researchers or medical professionals explain the purpose, procedures, risks, and benefits to the participants. Question and answer: Participants are encouraged to ask questions and seek clarification. Time for decision-making: Participants are given adequate time to consider their options before providing consent. | ||
| 5 | ||
| Special Considerations | ||
|---|---|---|
| Vulnerable populations: Extra care must be taken when obtaining consent from minors, individuals with cognitive impairments, or those in vulnerable situations. Language and cultural considerations: Information should be provided in a language and format that participants can understand. Confidentiality and privacy: Participants must be assured that their personal information will be kept confidential. | ||
| 6 | ||
| Informed Consent in Research | ||
|---|---|---|
| In research, informed consent is obtained before participants are enrolled in a study. Research protocols must be reviewed and approved by an ethics committee or institutional review board (IRB). Participants have the right to withdraw their consent at any time without facing any negative consequences. | ||
| 7 | ||
| Informed Consent in Medical Settings | ||
|---|---|---|
| In medical settings, informed consent is obtained before any treatment or procedure. Healthcare professionals must explain the nature of the treatment, potential risks, benefits, and alternatives. In emergencies, informed consent may be waived if immediate intervention is necessary to save a patient's life. | ||
| 8 | ||
| Ethical Considerations | ||
|---|---|---|
| Informed consent is based on the ethical principles of autonomy, beneficence, and respect for persons. Researchers and medical professionals have a duty to protect the rights and welfare of participants. Informed consent fosters trust, transparency, and ethical practice in research and medical settings. | ||
| 9 | ||
| Conclusion | ||
|---|---|---|
| Informed consent is a vital process that upholds ethical standards and protects the rights of individuals. It ensures that participants have the necessary information to make informed decisions about research or medical procedures. By obtaining informed consent, researchers and medical professionals promote trust, respect, and responsible practice. | ||
| 10 | ||