IR Spectroscopy Presentation
| Introduction to IR Spectroscopy | ||
|---|---|---|
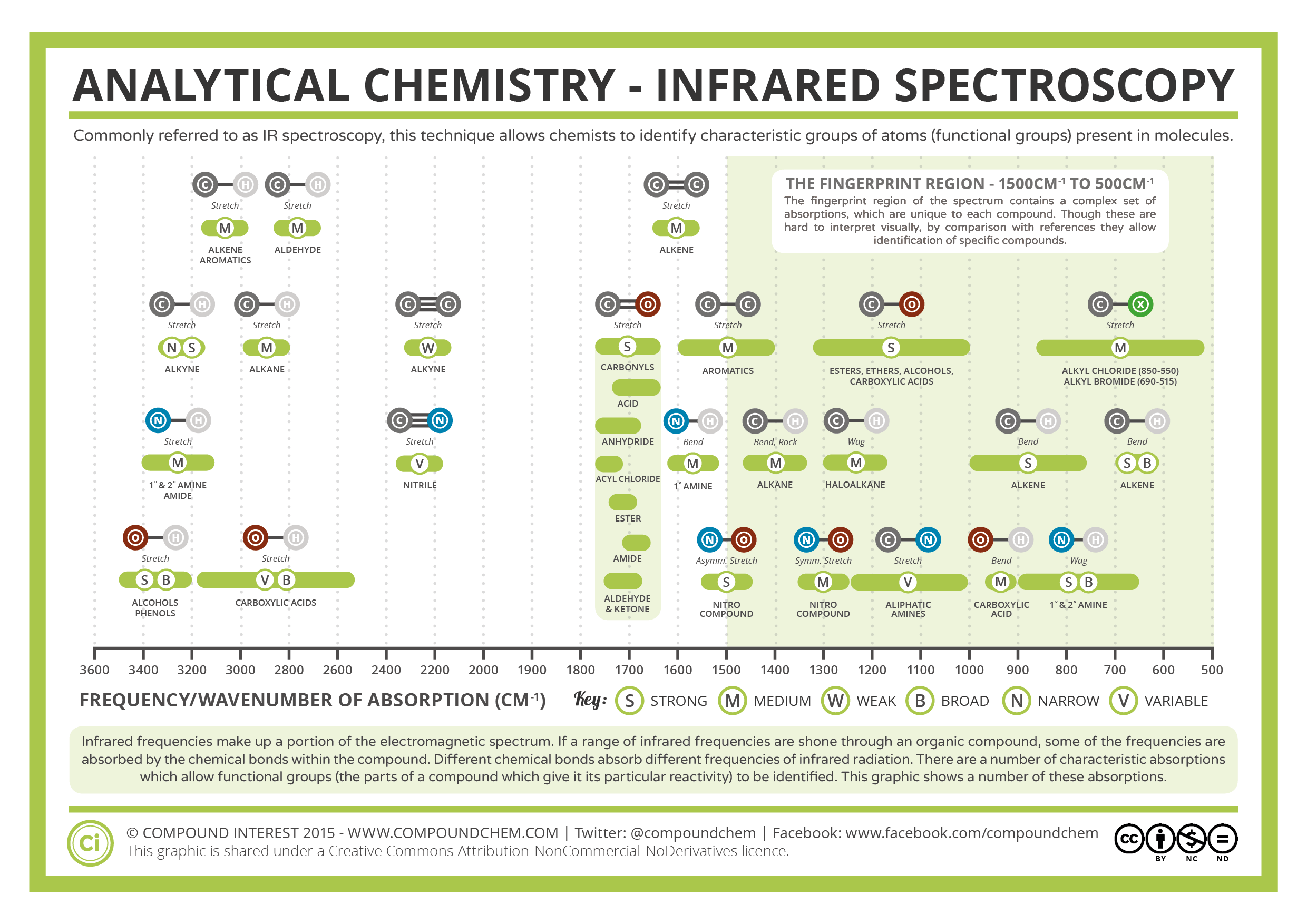
| IR Spectroscopy is a powerful analytical technique used to identify and characterize chemical compounds based on their interaction with infrared light. Infrared light lies between the visible and microwave regions of the electromagnetic spectrum. IR spectroscopy provides valuable information about the functional groups present in a compound, aiding in compound identification. | ||
| 1 | ||
| Principles of IR Spectroscopy | ||
|---|---|---|
| IR spectroscopy is based on the concept of molecular vibrations. When a molecule absorbs infrared light, it undergoes changes in vibrational and rotational energy, leading to characteristic absorption bands in the spectra. Different functional groups exhibit unique absorption frequencies, allowing for compound identification. | ||
| 2 | ||
| Instrumentation for IR Spectroscopy | ||
|---|---|---|
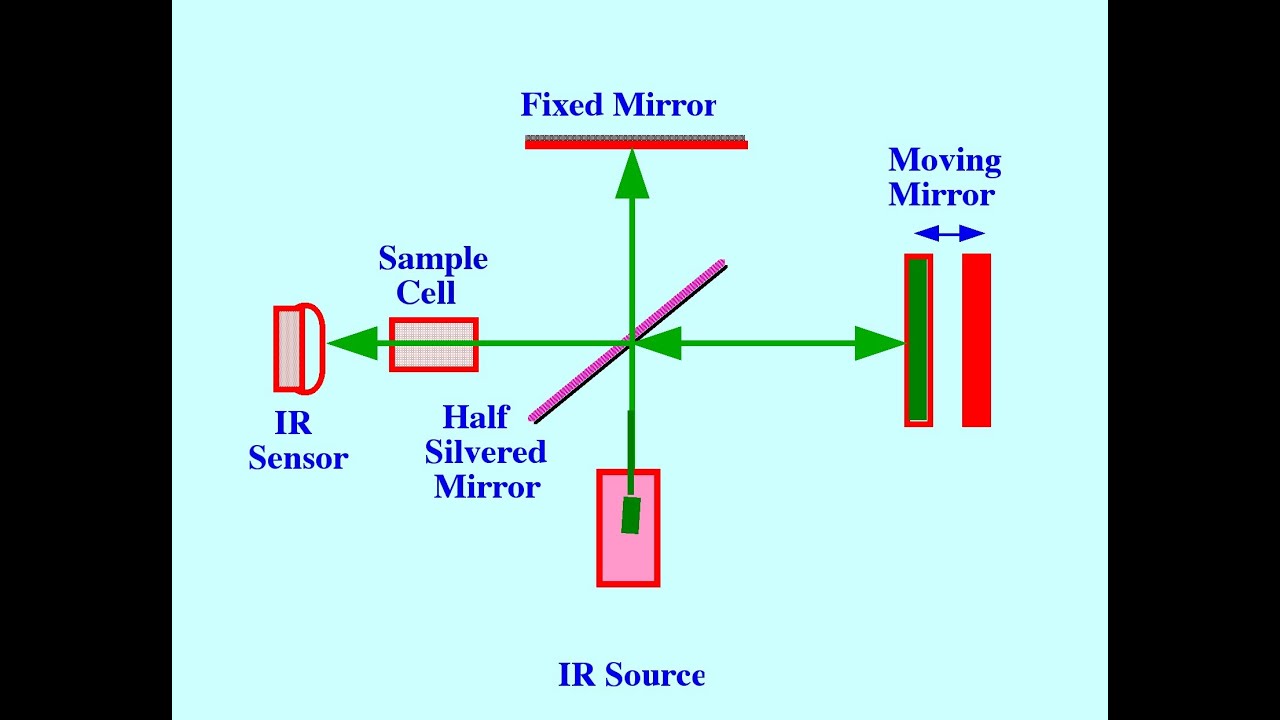
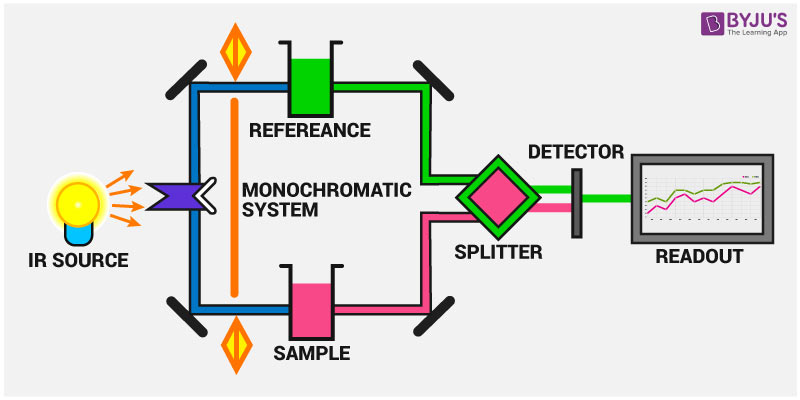
| IR spectrometers consist of a light source, sample holder, monochromator, and detector. Common light sources include Nernst glowers or globars, which emit infrared radiation. The sample is typically placed in a cell or on a salt plate and exposed to the infrared light. The transmitted or absorbed light is then detected. | ||
| 3 | ||
| Types of IR Spectroscopy | ||
|---|---|---|
| Fourier Transform Infrared Spectroscopy (FTIR) is the most widely used technique in IR spectroscopy due to its high sensitivity and rapid data acquisition. Attenuated Total Reflectance (ATR) is a sampling technique that allows for direct analysis of solid or liquid samples without extensive sample preparation. Near-Infrared Spectroscopy (NIR) is a related technique that utilizes a slightly higher energy range of infrared light and is often used for quantitative analysis. | ||
| 4 | ||
| Applications of IR Spectroscopy | ||
|---|---|---|
| IR spectroscopy finds applications in various fields such as pharmaceuticals, forensic analysis, food and beverage industry, and environmental monitoring. It is widely used for compound identification, purity analysis, and monitoring chemical reactions. IR spectroscopy can also be used to study biomolecules, such as proteins and DNA, providing insights into their structure and conformation. | ||
| 5 | ||
| Advantages and Limitations of IR Spectroscopy | ||
|---|---|---|
| Advantages: Non-destructive, requires minimal sample preparation, provides valuable information about functional groups and compound structure, and can be used for both qualitative and quantitative analysis. Limitations: Limited sensitivity for low-concentration samples, inability to differentiate between enantiomers, and interference from water vapor and carbon dioxide in the atmosphere. Note: This is a brief overview of IR spectroscopy. Further details and illustrations can be added to enhance the presentation. Your third bullet |  | |
| 6 | ||