Galvonic Corrosion Presentation
| Introduction to Galvanic Corrosion | ||
|---|---|---|
| Galvanic corrosion is a type of corrosion that occurs when two different metals are in contact with each other in the presence of an electrolyte. It is also known as bimetallic corrosion or dissimilar metal corrosion. The corrosion process is accelerated due to the electrochemical potential difference between the two metals. | ||
| 1 | ||
| Mechanism of Galvanic Corrosion | ||
|---|---|---|
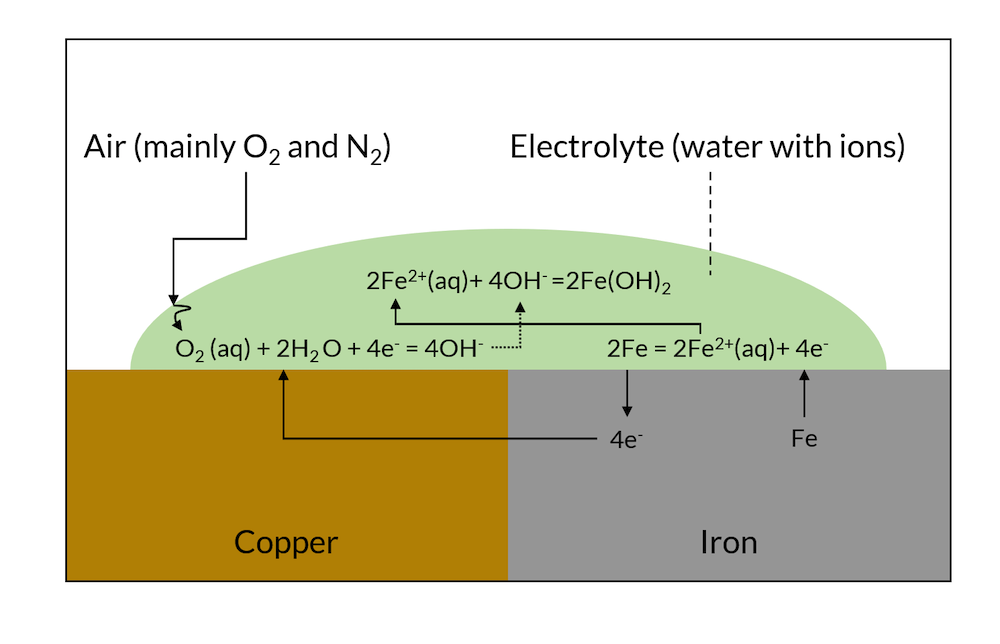
| Galvanic corrosion involves the transfer of electrons from the more electronegative metal (anode) to the less electronegative metal (cathode). The anodic metal undergoes oxidation and releases metal ions into the electrolyte. The cathodic metal acts as a reduction site for the dissolved metal ions, resulting in the deposition of corrosion products. | ||
| 2 | ||
| Factors Influencing Galvanic Corrosion | ||
|---|---|---|
| Electrochemical potential difference between the two metals: Greater potential difference leads to more severe galvanic corrosion. Surface area ratio: A larger surface area of the anodic metal accelerates the corrosion rate. Conductivity of the electrolyte: Higher conductivity promotes faster electron transfer and corrosion. | ||
| 3 | ||
| Effects of Galvanic Corrosion | ||
|---|---|---|
| Surface pitting and loss of material: Galvanic corrosion can cause localized damage, leading to pits and holes on the metal surfaces. Structural degradation: Corrosion can weaken the structural integrity of components, compromising their functionality and safety. Increased maintenance and repair costs: Galvanic corrosion necessitates frequent inspections, repairs, and replacements, adding to maintenance expenses. | ||
| 4 | ||
| Preventing Galvanic Corrosion | ||
|---|---|---|
| Proper material selection: Choosing metals with similar electrochemical potentials reduces the potential for galvanic corrosion. Isolation methods: Using insulating materials or applying protective coatings between dissimilar metals can prevent direct contact and minimize galvanic corrosion. Cathodic protection: Applying sacrificial anodes or impressed current systems can provide a more active cathode, protecting the anodic metal from corrosion. | ||
| 5 | ||
| Conclusion | ||
|---|---|---|
| Galvanic corrosion is a significant concern in various industries, including marine, aerospace, and construction. Understanding the mechanism, factors influencing it, and preventive measures is essential for mitigating the effects of galvanic corrosion. By implementing proper corrosion prevention strategies, the lifespan and performance of metal components can be significantly extended. | ||
| 6 | ||

_1516081191_20707-17.jpg)