Einstein Theory Of Specific Heat Presentation
| Introduction to Einstein Theory of Specific Heat | ||
|---|---|---|
| Einstein's theory of specific heat revolutionized our understanding of how heat is absorbed and released by solids. This theory was proposed by Albert Einstein in 1907 and provides a quantum mechanical explanation for specific heat. Specific heat is a measure of how much heat energy is required to raise the temperature of a substance by a certain amount. | ||
| 1 | ||
| Key Concepts in Einstein Theory of Specific Heat | ||
|---|---|---|
| According to Einstein's theory, atoms in a solid can only vibrate at specific frequencies or energy levels, called quanta. These quanta are discrete and quantized, meaning they can only have certain values and cannot take on any arbitrary energy. The specific heat of a solid is related to the average energy of these quanta and how they distribute among the atoms in the material. | ||
| 2 | ||
| The Einstein Model | ||
|---|---|---|
| The Einstein model assumes that all atoms in a solid vibrate at the same frequency, which is a simplification but provides useful insights. In this model, the energy of each atom is quantized into discrete energy levels, and the distribution of these energy levels follows a Boltzmann distribution. The average energy of an atom in the Einstein model is given by the Einstein energy, which is proportional to the temperature of the solid. | ||
| 3 | ||
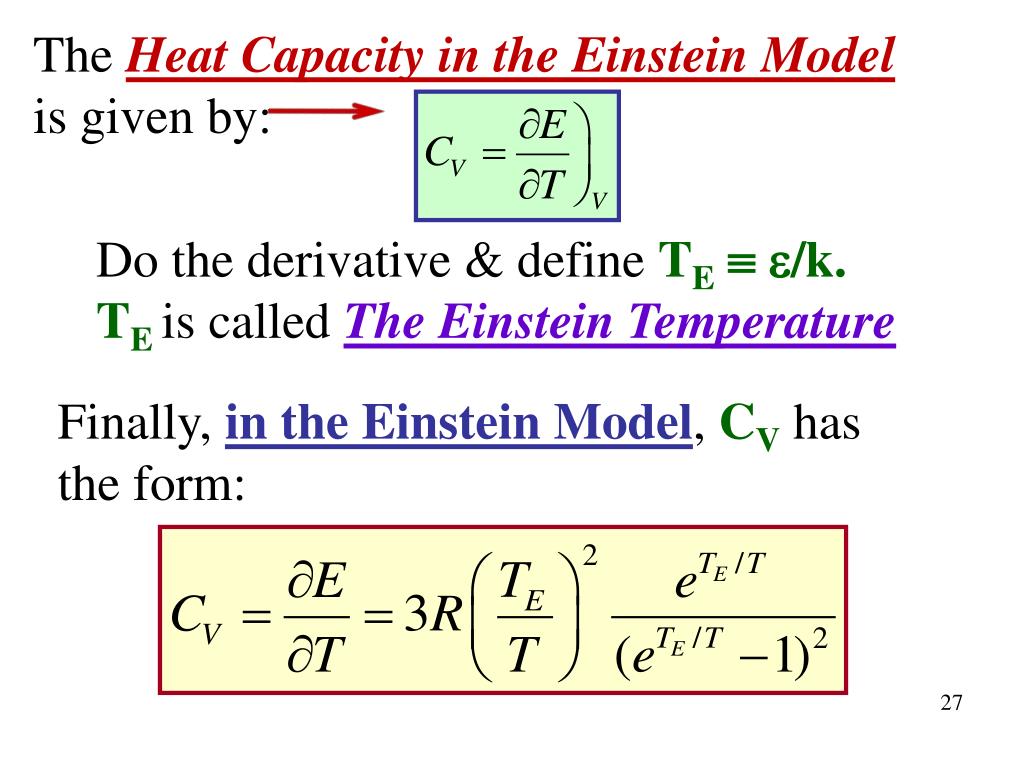
| Calculating Specific Heat using Einstein Theory | ||
|---|---|---|
| The specific heat of a solid can be calculated using the Einstein theory by considering the average energy of the atoms and the number of atoms in the material. The specific heat at constant volume, Cv, is given by Cv = 3Nk, where N is the total number of atoms and k is the Boltzmann constant. This equation shows that the specific heat is directly proportional to the number of atoms and the Boltzmann constant. | ||
| 4 | ||
| Limitations of the Einstein Theory | ||
|---|---|---|
| The Einstein theory of specific heat is an approximation and does not take into account the interactions between atoms in a solid. It assumes that all atoms are independent and do not interact with each other, which is not always true in real materials. Therefore, the Einstein model is most accurate for materials with weak interatomic interactions, such as noble gases. | ||
| 5 | ||
| Applications of Einstein Theory | ||
|---|---|---|
| Despite its limitations, the Einstein theory of specific heat has been successfully applied to various materials, including metals, insulators, and gases. It provides a framework for understanding the temperature dependence of specific heat and has been instrumental in the development of thermodynamic models. Additionally, the Einstein theory has found applications in fields such as materials science, solid-state physics, and quantum mechanics. | ||
| 6 | ||
| Conclusion | ||
|---|---|---|
| Einstein's theory of specific heat offers a quantum mechanical explanation for the temperature dependence of specific heat in solids. It provides insights into the behavior of atoms in a solid and has been widely used to calculate specific heat in various materials. While the Einstein model has its limitations, it remains a valuable tool in the study of thermodynamics and materials science. | ||
| 7 | ||