DE BROGLIE HYPOTHESIS Presentation
| Introduction to the De Broglie Hypothesis | ||
|---|---|---|
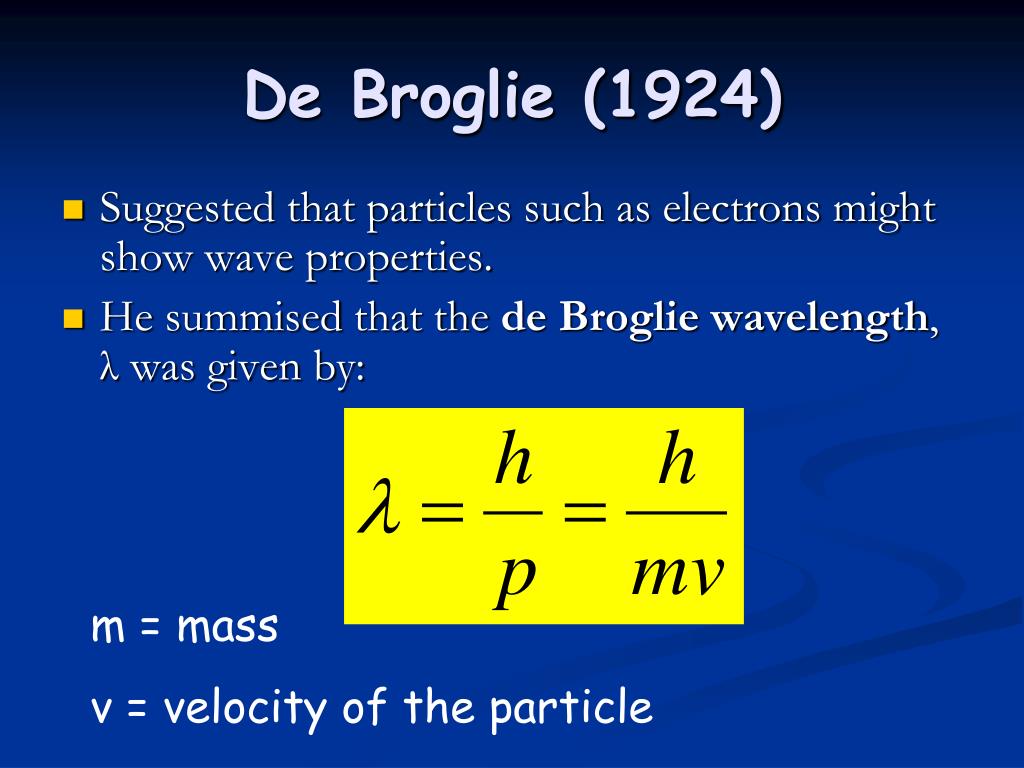
| The De Broglie Hypothesis states that particles, such as electrons, have wave-like properties. Proposed by Louis de Broglie in 1924, it extends the wave-particle duality concept of quantum mechanics. According to the hypothesis, every particle with momentum has an associated wavelength. | ||
| 1 | ||
| Particle-Wave Duality | ||
|---|---|---|
| The wave-particle duality concept suggests that particles can exhibit both wave and particle-like behavior. De Broglie's hypothesis built upon this idea by proposing that particles also have wave properties. It implies that particles can interfere with each other, just like waves do. | ||
| 2 | ||
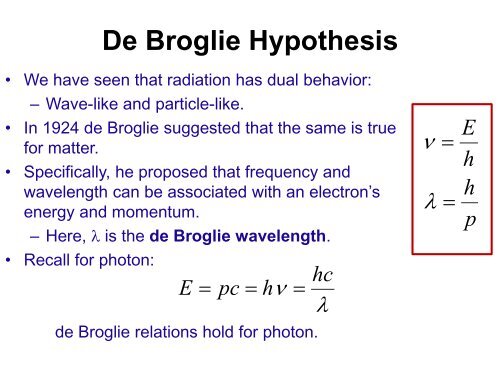
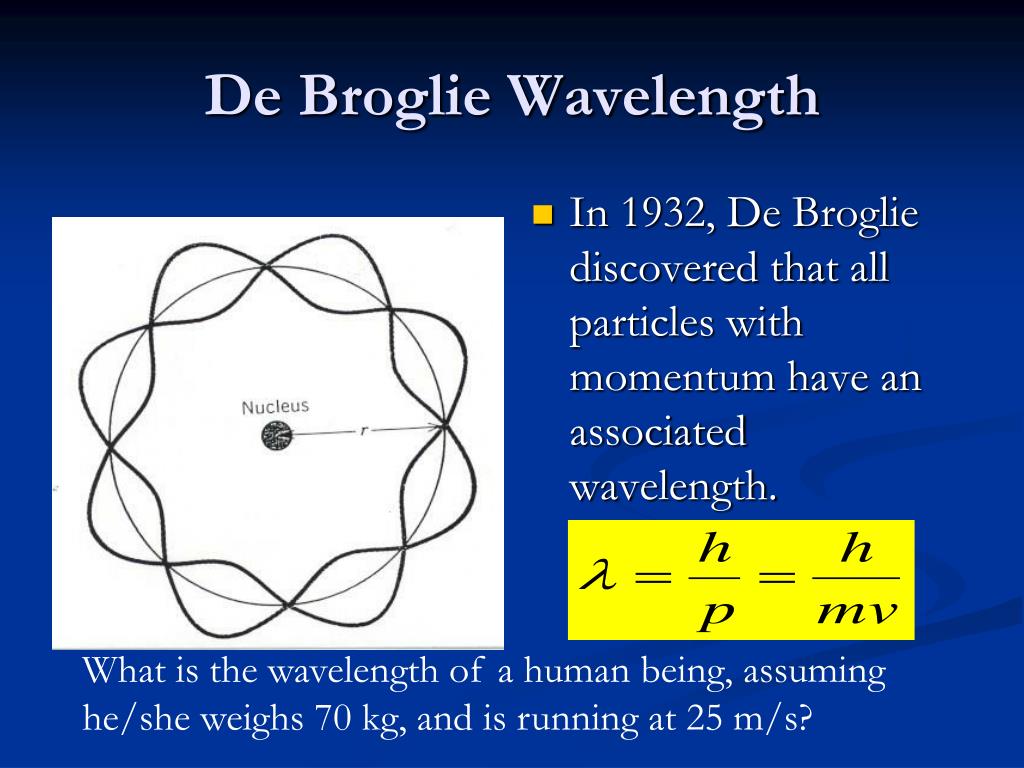
| Equation for De Broglie Wavelength | ||
|---|---|---|
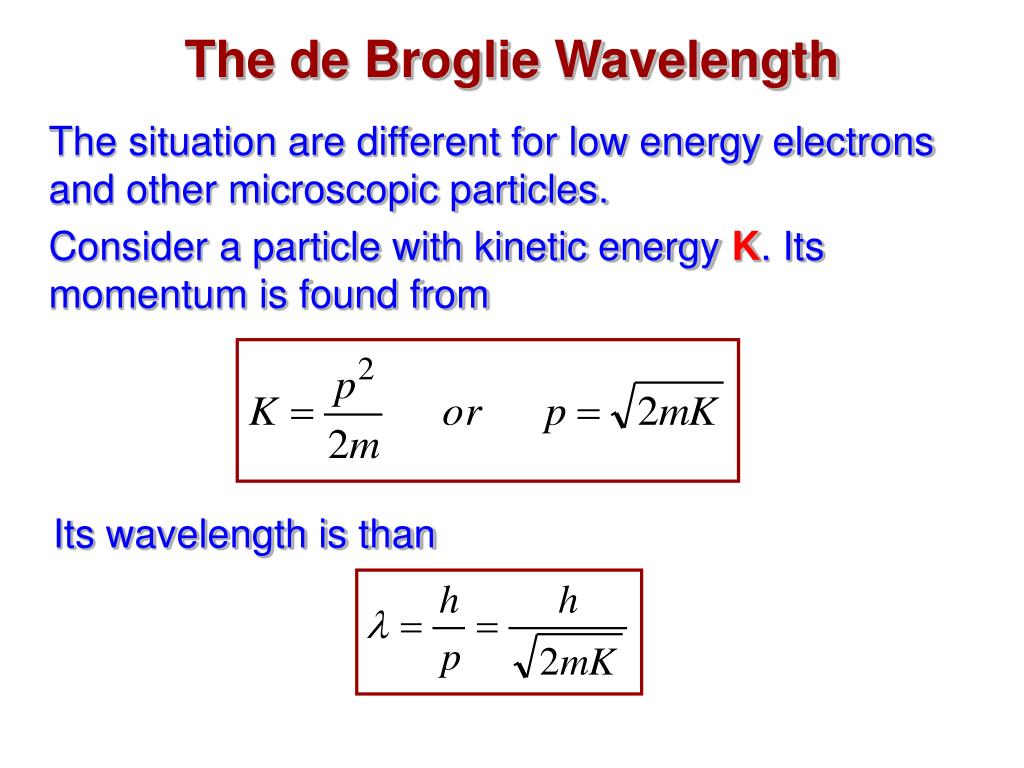
| The De Broglie wavelength (λ) of a particle is given by the equation: λ = h / p λ represents the wavelength, h is Planck's constant, and p denotes the momentum of the particle. The equation shows that the wavelength decreases as the momentum of the particle increases. | ||
| 3 | ||
| Experimental Confirmation | ||
|---|---|---|
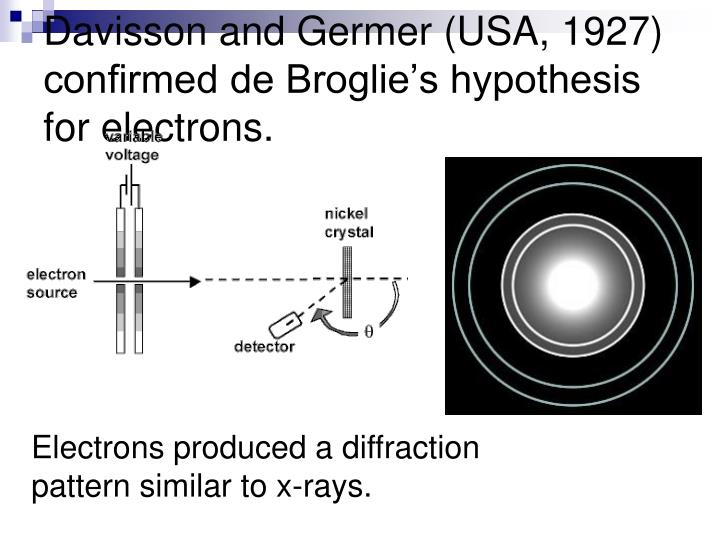
| The De Broglie Hypothesis was experimentally confirmed by Davisson and Germer in 1927. They observed electron diffraction, which is the bending of electron beams in a crystal lattice. This diffraction pattern supported the wave-like nature of electrons proposed by de Broglie. | ||
| 4 | ||
| Applications of De Broglie Hypothesis | ||
|---|---|---|
| The De Broglie Hypothesis is fundamental to understanding quantum mechanics. It explains phenomena such as electron diffraction, which is used in electron microscopy. The hypothesis also plays a crucial role in particle accelerators and quantum computing. | ||
| 5 | ||
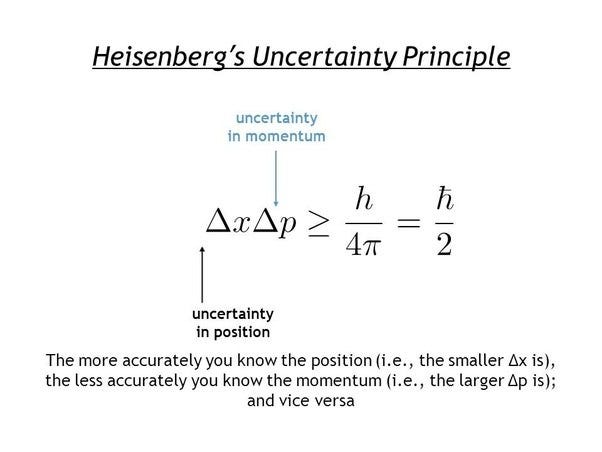
| Relation to Heisenberg's Uncertainty Principle | ||
|---|---|---|
| De Broglie's hypothesis is closely related to Heisenberg's Uncertainty Principle. The Uncertainty Principle states that the position and momentum of a particle cannot be precisely determined simultaneously. De Broglie's wavelength provides a connection between the uncertainty in momentum and the spatial extent of the wave function. | ||
| 6 | ||
| Limitations of De Broglie Hypothesis | ||
|---|---|---|
| The De Broglie Hypothesis is valid for particles with non-relativistic velocities. It fails to accurately describe the behavior of particles traveling at relativistic speeds. For relativistic particles, the more comprehensive theory of quantum field theory is used. | ||
| 7 | ||
| Significance in Modern Physics | ||
|---|---|---|
| The De Broglie Hypothesis paved the way for the development of quantum mechanics. It provided a crucial understanding of the wave-particle duality of matter. The hypothesis revolutionized our understanding of the behavior of particles at the atomic and subatomic levels. | ||
| 8 | ||
| De Broglie's Contribution to Science | ||
|---|---|---|
| De Broglie's work on the wave-particle duality earned him the Nobel Prize in Physics in 1929. His hypothesis was a significant step towards the development of quantum mechanics. De Broglie's groundbreaking ideas continue to shape our understanding of the microscopic world. | ||
| 9 | ||
| References (download PPTX file for details) | ||
|---|---|---|
| Davisson, C., & Germer, L. H. (1927). Diffrac... De Broglie, L. (1925). Recherches sur la théo... Griffiths, D. J. (2005). Introduction to quan... |  | |
| 10 | ||
| References (download PPTX file for details) | ||
|---|---|---|
| Your first bullet... Your second bullet... Your third bullet... |  | |
| 11 | ||