Accelerated Stability Studies Presentation
| Introduction to Accelerated Stability Studies | ||
|---|---|---|
| Accelerated stability studies are conducted to determine the stability of a product under accelerated conditions. These studies are used to predict the shelf life of a product and assess its quality over time. Accelerated stability studies involve subjecting the product to elevated temperatures and humidity levels. | ||
| 1 | ||
| Purpose of Accelerated Stability Studies | ||
|---|---|---|
| The main purpose of accelerated stability studies is to shorten the time required to assess product stability. These studies help determine the effect of temperature and humidity on the product's physical, chemical, and microbiological properties. Accelerated stability studies provide valuable information for product formulation, packaging, and storage recommendations. | ||
| 2 | ||
| Study Design and Conditions | ||
|---|---|---|
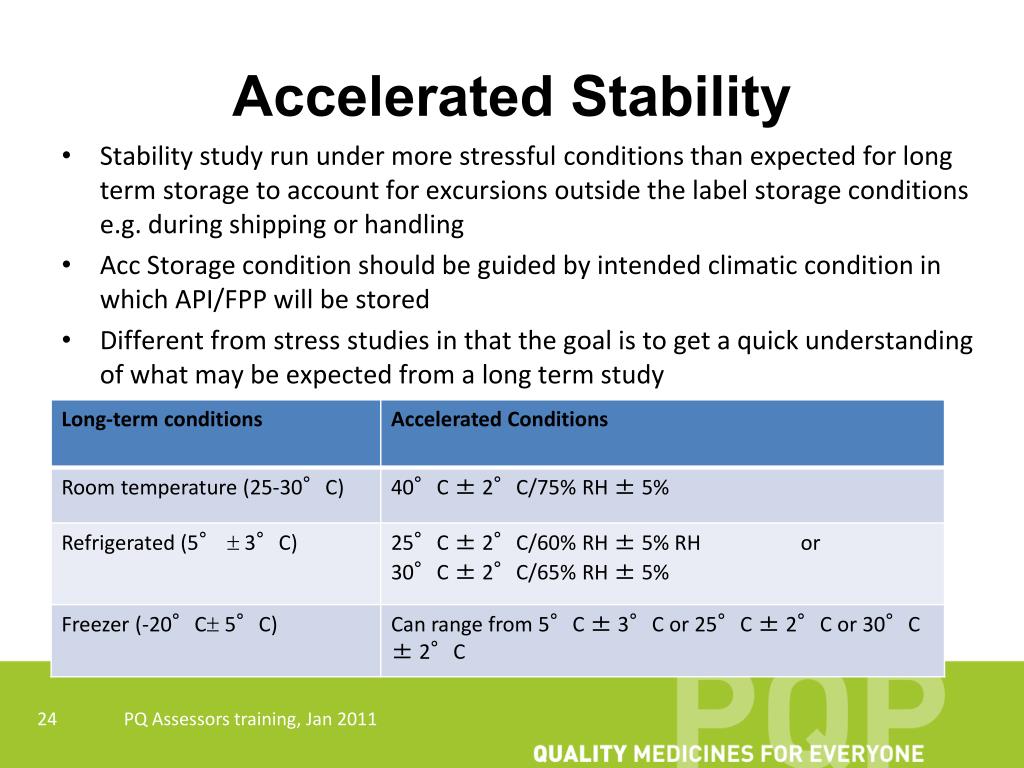
| The study design for accelerated stability studies typically follows guidelines provided by regulatory agencies. Accelerated conditions may include elevated temperatures (e.g., 40°C, 50°C, or higher) and humidity levels (e.g., 75% RH). The duration of the study is usually shorter than real-time stability studies, ranging from weeks to months. |  | |
| 3 | ||
| Evaluation Parameters | ||
|---|---|---|
| Physical parameters such as appearance, color, odor, and texture are evaluated during accelerated stability studies. Chemical parameters including pH, assay, degradation products, and impurities are also assessed. Microbiological parameters, such as microbial growth and preservative effectiveness, are examined as well. | ||
| 4 | ||
| Data Analysis and Interpretation | ||
|---|---|---|
| The data obtained from accelerated stability studies is analyzed to determine any changes in the product over time. Statistical methods are applied to assess the significance of observed changes and to estimate shelf life. Interpretation of the data helps in making informed decisions about product formulation, packaging, and storage conditions. | ||
| 5 | ||
| Limitations of Accelerated Stability Studies | ||
|---|---|---|
| Accelerated stability studies may not fully replicate the actual conditions experienced during real-time storage. The results obtained from accelerated studies are extrapolated and should be interpreted with caution. Some degradation mechanisms may not be adequately accelerated, leading to underestimation or overestimation of product stability. | ||
| 6 | ||
| Regulatory Requirements | ||
|---|---|---|
| Regulatory agencies, such as the FDA and ICH, provide guidelines on accelerated stability studies for different product categories. These guidelines outline the study design, conditions, evaluation parameters, and acceptance criteria. Compliance with regulatory requirements is essential for product approval and market authorization. | ||
| 7 | ||
| Importance of Accelerated Stability Studies | ||
|---|---|---|
| Accelerated stability studies provide critical information for product development, formulation optimization, and quality control. These studies help identify potential stability issues early in the product life cycle, allowing for timely corrective actions. Accelerated stability data supports regulatory submissions and helps establish product shelf life and storage conditions. | ||
| 8 | ||
| Case Study: Accelerated Stability Studies for a Pharmaceutical Product | ||
|---|---|---|
| A case study showcasing the application of accelerated stability studies in pharmaceutical product development and regulatory approval. The study involved subjecting the product to elevated temperatures and humidity levels and assessing its stability parameters. The data obtained from the study supported the determination of shelf life and storage conditions for the product. | ||
| 9 | ||
| Conclusion | ||
|---|---|---|
| Accelerated stability studies play a crucial role in assessing the stability and quality of products. These studies provide valuable information for product formulation, packaging, and storage recommendations. Compliance with regulatory guidelines and careful interpretation of data are essential for successful accelerated stability studies. | ||
| 10 | ||